Customer procedure for importing ITE and Medical power supplies/AC adaptors to Japan
The Japan PSE Law and its regulations specify mandatory electrical safety and EMI requirements for electrical products sold in Japan. Products with a history of accidents in the marketplace, or products which are likely to cause injury, are termed “Specified Electrical Products” and require third-party assessment by a METI-Registered Conformity Assessment Body (Registered CAB). Specified Electrical Products must display the diamond PSE mark. Power supplies and AC Adapters are “Specified electrical products” and must have a PSE mark.
An overview of the regulation is available online: https://www.meti.go.jp/policy/consumer/seian/denan/file/06_guide/denan_guide_ver3_en.pdf
Obligations/Procedure for Importers of Globtek Power supplies into Japan
- Importers are obligated to submit an official notification to METI prior to importing electrical equipment subject to the PSE Law (Article 3, Notification of Business Commencement)
- Importers shall Obtain an “authorized valid copy” of the METI-designated CAB’s PSE Certificate from GlobTek and keep the original on file in Japan
- Apply a label over the GlobTek PSE label with PSE label showing actual importer name. (See below diagram)
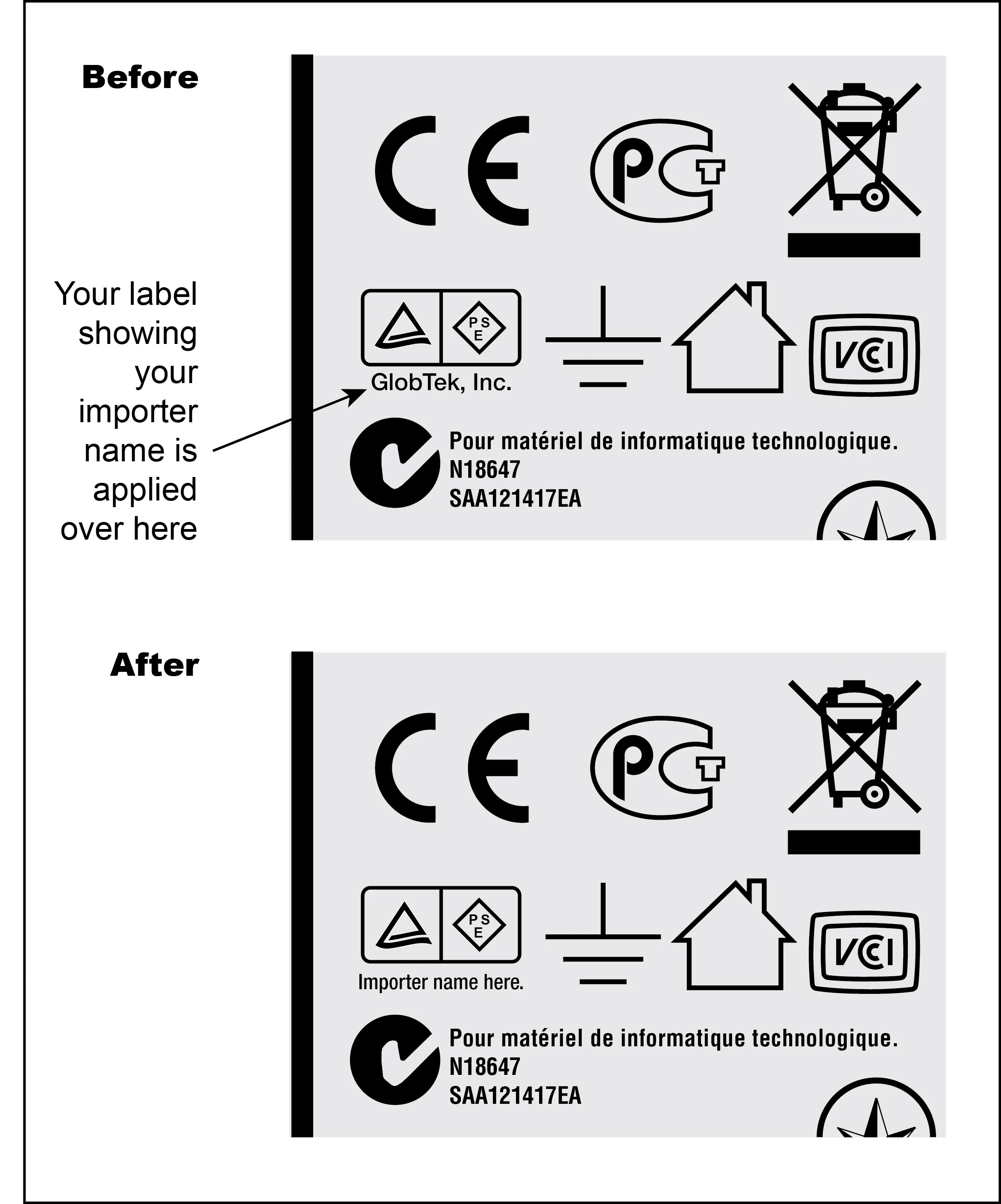
View a real time listing of PSE Approved Medical (60601-1) Power Supply/AC Adapters that meet Japan DENAN regulations for import and sale in Japan